Oliver Baker
he/him | age 17 | Kentville, NS
2021 AVRSF Gold Medal | 2021 CWSF Bronze Medal
Edited by Iman Kashif
INTRODUCTION
Sampling to detect plastic is important for the health of the ocean, as ingesting them can cause harm to the biota it contains (Andrady, 2015; Paul-Pont et al., 2016). Microplastics are pieces of plastic that range from 0.1 to 5,000 μm (CONTAM, 2016). They enter the ocean as debris and are broken down into their current form.
Mussels (Mytilus) are a marine invertebrate found all over the world and can contain microplastics (Browne et al., 2008; Ding et al., 2018; Li et al., 2019; Lusher et al., 2017; Mathalon and Hill, 2014). Blue mussels (Mytilus edulis) are important to examine because they are consumed by humans. They are useful for identifying plastics due to their susceptibility to uptake microplastics (Li et al., 2019). By identifying where microplastics are being ingested by mussels, then there could be potential for them to be in that area (Brown et al., 2008).
The purpose of this study was to investigate whether blue mussels off of southern Nova Scotia are accumulating microplastics in their tissue and if so, explore if they can be used as bioindicators of plastic contamination in the water. It was hypothesized that the mussels would be consuming microplastics off the coast of Southern Nova Scotia. Previous studies have demonstrated that they have been bioaccumulating in Mytilus edulis in the Halifax Harbour (Mathalon and Hill, 2014), as well as in the LaHave Estuary (Coastal Action, 2018).
MATERIALS & METHODS
Ten unprocessed blue mussels were collected from a mussel farmer in Lunenburg County on November 21, 2020, and on January 20, 2021. Once collected, the mussels were put in a freezer and frozen at -15° C for up to 68 days.
The mussels were defrosted, measured and weighed (Figure 1). All of the tools and containers were cleaned with double distilled water before analysis. Mussel tissue was extracted and placed in a glass test tube. The mussels were stored at -20 °C between analyses.
Potassium Hydroxide (KOH) was used to digest the tissue of the mussels (Lusher et al., 2017). 20 ml of a prepared 10% KOH solution was added to each test tube using a serological pipette (Figure 1). A control was prepared using the KOH from each solution. The test tubes were placed in an incubator for twenty-four hours at 40 °C (Thiele et al., 2019).
Following incubating the test tubes, they were mixed by inverting them. They were then vacuum filtered through 20 μm and 7 μm filter paper (Figure 1). Each filter paper was placed on an individual Petri dish, covered with a lid and was examined under a microscope (Figure 1).
The microplastics were identified by eye (Lusher et al., 2017). The objects viewed had to be homogeneous in colour, have no organic structures, and have an equal thickness (Hidalgo-Ruz et al., 2012; Lusher et al., 2015). All suspected microplastics were photographed (Figure 2).
Figure 1: A flowchart showing steps used to digest the mussel tissue and look for microplastics.
Figure 2: A picture of a blue microplastic fibre in the second control sample under 320x magnification.
RESULTS
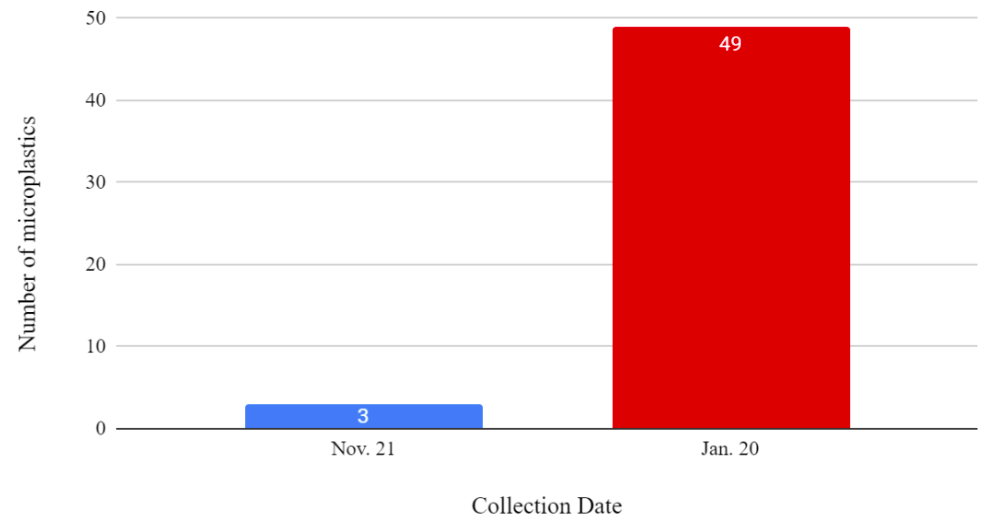
A total of 133 microplastic particles were found (Figure 3). There were 46 microplastics found in the mussels from November and 87 microplastics found in the mussels from January (Figure 3). Figure 3 is done without the number of microplastics in the controls being subtracted because the possibility of microplastic contamination in the controls was explored.
Both controls had multiple microplastics found. Eight particles were found in the first control, and four were found in the second control. The number of microplastics found in the control was subtracted in Figure 4. The control shows that microplastics are found in the lab.
Figure 3: A histogram showing the collection date and the number of microplastics in the mussels without the control being subtracted.
Figure 4: A histogram showing the date of collection and the number of microplastics in the mussels with the control being subtracted.
DISCUSSION
The results from this study have shown that there are microplastics in blue mussels in Lunenburg County, Nova Scotia and that the number increased over two months. The microplastics in the mussels were quantified; however, the numbers differ from other studies in the area. Mathalon and Hill (2014), a nearby study, found numbers in the hundreds. They also had microplastics in their control, suggesting that microplastics could contaminate the control of a plastic study and affect the results. Additionally, this provides insight into the numbers of microplastics present in the environment because of the plastic found in a sample that should have had none.
The control from this study was made of 10% KOH and only came into contact with a double-rinsed glass jar and double-distilled water, but both controls still contained microplastics. Contamination was mitigated by following precautions, such as rinsing all the equipment with double distilled water before use, using nitrile gloves and wearing a laboratory coat (Davison & Asch, 2011; Lusher et al., 2015; Mathalon & Hill, 2014). These precautions mitigate airborne filaments that could cause contamination. Because there was microplastic contamination in the control, it has made it difficult to accurately quantify the amount found.
A limitation of this study is the verification of microplastics because there is a 70% potential error rate when only using visual identification (Lusher et al., 2017). For example, there were other sediments on the 20 μm filter paper. The sediments were typically larger than the microplastics, which could mean that the sediments could have been concealing smaller pieces underneath them. Additionally, there were pieces of the 20 μm filter paper that showed up on the 7 μm filter paper. The pieces of the 20 μm paper had a transparent colour that resembled microplastics, but their rough texture made them not identifiable as one. However, there was a potential misidentification of these pieces as microplastics. To verify the identified particles, a chemical analysis could be done to identify if a particle was made of plastic or not, which can be done using Fourier-red transform spectroscopy (Hidalgo-Ruz et al., 2012; Lusher et al., 2017; Mathalon and Hill, 2014). To make reliable statistics of the presence of microplastics, infrared spectroscopy should be used in addition to visual identification.
There was a potential loss of microplastics throughout the process of analysis (Lusher et al., 2017). The pressure of the vacuum that filtered the mussel tissue and KOH created air bubbles from being on an unavoidable suboptimal power. The bubbles were diluted to remove any potential microplastics in the test tube, although some of the bubbles and sediments remained.
CONCLUSION
Contrarily, the results of this study show that blue mussels show that there are microplastics in the environment and that Mytilus edulis could be used as a bioindicator for plastic contamination. Some mussels had more than the number of microplastics found in the control, which suggests that the waterway in which they live is contaminated with plastic. Furthermore, ingested mussels from this area would result in the ingestion of microplastics, which is harmful to human health (Smith et al., 2018). By quantifying microplastics in blue mussels, the number of them a human could safely consume could be quantified and assessed for harmful quantities.
Future research could be explored during the winter to determine if microplastic presence is larger in the winter months than in the summer. This would determine the time of the year that would be best to consume mussels, as they could contain a different number. This study collected mussels in the winter months and showed less of a presence of microplastics than a study less than 100 kilometres away (Mathalon and Hill, 2014).
Studies that quantify microplastics in organisms are important to determine the threat marine invertebrates and humans face from the increasing problem of plastic contamination in the ocean. This study confirmed that there are microplastics in blue mussels and that they are an entry point into humans.
ACKNOWLEDGEMENTS
I would like to thank Clare Kellock and Julia LeBlanc, from Coastal Action, for providing me with guidance and financial support as part of the Nova Action Cohort. The mussel farmer who provided the mussels is greatly appreciated. I am grateful to my mentors, Russell Easy, Ph.D., MSc, and Laura Ferguson, Ph.D., MSc, for the help and access to Dr. Easy’s lab at Acadia.
REFERENCES
Andrady, A. L. (2015, June 2). Persistence of Plastic Litter in the Oceans. Marine Anthropogenic Litter, 57-72. Retrieved from https://doi.org/10.1007/978-3-319-16510-3_3
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., & Thompson, R. C. (2008, May 30). Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environmental Science & Technology, 42(13), 5026-5031. Retrieved from https://doi.org/10.1021/es800249a
Coastal Action. (2018). Atlantic Canada Microplastic Research Project. Coastal Action. Retrieved from https://www.coastalaction.org/uploads/1/2/2/2/122203881/atlantic_canad a_microplastic_research_project_-_2018_surface_water_report_-_may_8.pdf
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011, December). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62(12), 2588-2597. Retrieved from doi.org/10.1016/j.marpolbul.2011.09.025
Davison, P., & Asch, R. G. (2011, June 27). Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. MARINE ECOLOGY PROGRESS SERIES, 432, 173–180. DOI: 10.3354/meps09142
Ding, J.-F., Li, J.-X., Sun, C.-J., He, C.-F., Jiang, F.-H., Gao, F.-L., & Zheng, L. (2018, May). Separation and Identification of Microplastics in Digestive System of Bivalves. Chinese Journal of Analytical Chemistry, 46(5), 690-697. Retrieved from https://doi.org/10.1016/ S1872-2040(18)61086-2
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., & Thiel, M. (2012, February 9). Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. American Chemical Society, 46(6), 3060-3075. Retrieved from https://doi.org/10.1021/es2031505
Li, J., Lusher, A. L., Rotchell, J. M., Deudero, S., Turra, A., Brate, I. L. N., Sun, C., Hossain, M. S., Li, Q., Kolandhasamy, P., & Shi, H. (2019, January). Using mussel as a global bioindicator of coastal microplastic pollution. Environmental Pollution, 244, 522-533. Retrieved from https://doi.org/10.1016/j.envpol.2018.10.032
Lusher, A., Brate, I. L. N., Hurley, R., Iversen, K., & Olsen, M. (2017, May 12). Testing of methodology for measuring microplastics in blue mussels (Mytilus spp) and sediments, and recommendations for future monitoring of microplastics (R & D-project). NIVA. Retrieved from https://niva.brage.unit.no/niva-xmlui/bitstream/handle/11250/2470297/72 09-2017.pdf?sequence=1&isAllowed=y
Lusher, A. L., O'Donnell, C., Officer, R., & O'Connor, I. (2015, December 23). Microplastic interactions with North Atlantic mesopelagic fish. ICES Journal of Marine Science, 73(4), 1214–1225. Retrieved from https://doi.org/10.1093/icesjms/fsv241
Mathalon, A., & Hill, P. (2014, April 15). Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Marine Pollution Bulletin, 81(1), 69-79. Retrieved from https://doi.org/10.1016/j.marpolbul.2014.02.018
Panel on Contaminants in the Food Chain (CONTAM). (2016, June 23). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA, 14(6). Retrieved from https://doi.org/10.2903/j.efsa.2016.4501
Paul-Pont, I., Lacroix, C., Gonzalez, C. F., Hégaret, H., Lambert, C., Le Goïc, N., Frère, L., Cassone, A.-L., Sussarellu, R., Fabioux, C., Guyomarch, J., Albentosa, M., Huvet, A., & Soudant, P. (2016, September). Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environmental Pollution, 216, 724-737. Retrieved from https://doi.org/10.1016/j.envpol.2016.06.039
Smith, M., Love, D. C., Rochman, C. M., & Neff, R. A. (2018, August 16). Microplastics in Seafood and the Implications for Human Health. Curr Envir Health Rpt, 5, 375-386. Retrieved from https://doi.org/10.1007/s40572-018-0206-z
Thiele, C. J., Hudson, M. D., & Russell, A. E. (2019, May). Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Marine Pollution Bulletin, 142, 384-393. Retrieved from https://doi.org/10.1016/j.marpolbul.2019.03.003
ABOUT THE AUTHOR
Oliver Baker
Oliver Baker is a 17-year-old grade 12 French Immersion student from Kentville, Nova Scotia. He is passionate about the environment and the growing concerns of plastic pollution and sea-level rise. His two science fair projects have received medals at the national science fair. He enjoys track & field, ultimate frisbee, rowing, and soccer, as well as working towards his level 10 RCM in piano and playing in the Nova Scotia Youth Wind Ensemble. This keeps him busy, but he finds time to volunteer at school and within the community at local nursing homes and is an Outreach Ambassador at STEM Fellowship.